Extraction
of Caffeine/Coffee Oil Using Liquid-Liquid Extraction
Introduction
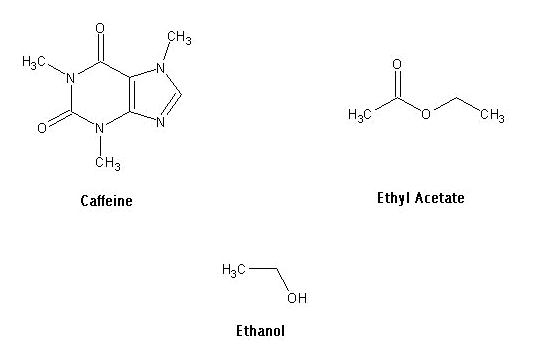
Caffeine is known medically as trimethylxanthine and
possesses the chemical formula C8H10N402. It
occurs naturally in over 60 plants, including coffee beans, tea leaves and cocoa
nuts. In its pure state, it exists
as a bitter white powder. Caffeine
is used in the medical field as well as food processes. Medically, caffeine is used as a cardiac stimulant and a mild
diuretic. In the food industry,
caffeine is marketed as a means to boost energy. It can be found in most type of colas, coffee, tea and
chocolate products. Caffeine is
also prevalent in over-the-counter stimulants, such as Vivarin and NoDoz.
The primary source of pure caffeine is the process of decaffeinating
coffee and tea.
Proposed
Design Problem & Solution
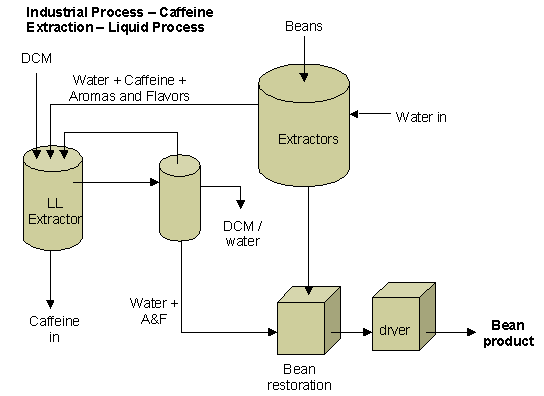
There are 2 methods of decaffeinating coffee, the
indirect and direct means. The
indirect means uses water as the decaffeinating agent.
It is passed through the coffee beans and released into a separate
chamber. There, it is
decaffeinated. The water is then
returned to the coffee to restore its natural flavor.
The direct method mixes an organic decaffeinating agent, such as
dichloromethane, ethyl acetate or supercritical carbon dioxide, directly with
the coffee. The caffeine binds to
this substance and then is removed from the coffee, usually through boiling.
The direct means of decaffeinating is the process we choose to replicate.
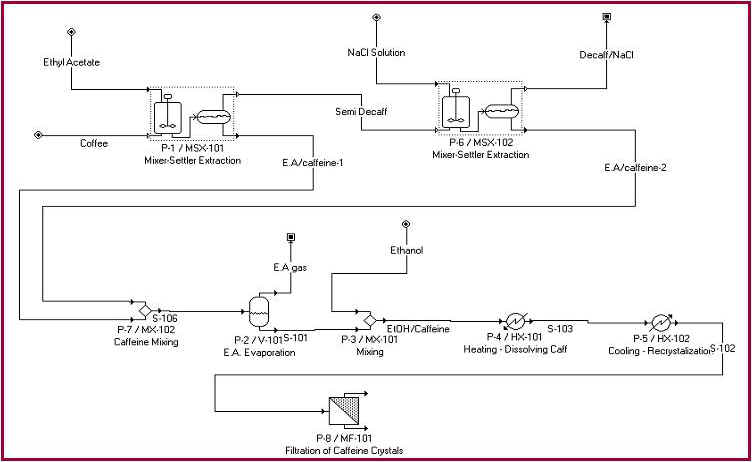
Detailed
Flow Chart from SuperPro
The various steps in the decaffeinating process can be
found in the SuperPro flowchart in Appendix A.
Literature
Review
Fourier
transform infrared determination of caffeine in roasted coffee samples
Fresenius
Journal of Analytical Chemistry (2000) 366: 319-322
Coffee contains several alkaloids, caffeine being the most important one.
For consumer information, it is required to accurately determine the
caffeine concentration in commercially roasted coffee.
A new procedure has been developed for the Fourier transform infrared
(FT-IR)
determination
of caffeine in roasted coffee samples.
It uses the same type of decaffeinating agents we used in our
experimental procedures. The CHCl3
extracted the caffeine from the wetted coffee samples.
The FT-IR procedure then measured absorbance to determine the
concentration of caffeine in the coffee. The
extraction of caffeine by organic solutions was successful in this study, which
furthered our hypothesis that caffeine could be obtained using this type of
process in our experimental process.
Effects
of caffeine, caffeine-associated stimuli, and caffeine-related information on
physiological and psychological arousal
Psychopharmacology
DOI 10.1007/s002132100841
Caffeine is a known stimulant. The
study conducted in this journal article tested the physiological and
psychological effects caffeine had on various individuals.
The caffeine-associated stimuli increased alertness, contentedness and
skin conductance levels. The
information that the drink contained caffeine alone decreased calmness in the
subjects. Decaffeinated drinks (including coffee) were placebos given
to subjects. After consumption,
increased contentedness was reported. The
authors concluded that the caffeine-associated stimuli increased arousal and
information about the content of the drink modulated arousal in the direction
indicated by the information. The
caffeine evidently effected the subjects and the time and research put into this
study show the demand for caffeine on the market as well as decaffeinated
products.
Mauldin,
R.F., Burns, D.J., Keller, I.K, Koehn, K.K., Johnson, M.J, Gray, S.L. Theory
of
Supercritical Fluid Extraction via the Discovery Approach Chem. Educator. 1999,
183-185.
Supercritical CO2 is a new technology that is being explored
for the use in extraction. It
involves high pressure CO2 which can be used to extract organic
compounds, like caffeine or nicotine. This
can be used to obtain a product, or be used to take the product out, like for
the decaffeination of coffee. This
is a very good alternative to other extraction methods because it is non-toxic
and easy to separate from the product of interest.
Sarmento,
M., Pires, M., Cabral, J., and Aires-Barros, M.
1997. Liquid-liquid
extraction of a recombinant protein, cytochrome b5, from an impure extract using
aqueous two-phase systems. Bioprocess
Engineering. 16: 295-297.
Liquid-liquid
extraction is used extensively in chemical and pharmaceutical industry, though
it was not until recently that this technique has been used to recover
bipolymers (proteins) because of their low solubility in organic solvents and
the denaturing effect the solvents have on the structure of the protein
A
two-phase system of polyethylene glycol and potassium phosphate salts were used
to perform a liquid-liquid extraction of cytochrome b5 from sheared Escherichia
coli cells. This extraction
process was a single step process that allowed the complete removal of cell
debris and a nearly 67% recovery of the target protein from the aqueous layer.
.
Experimental
Plan:
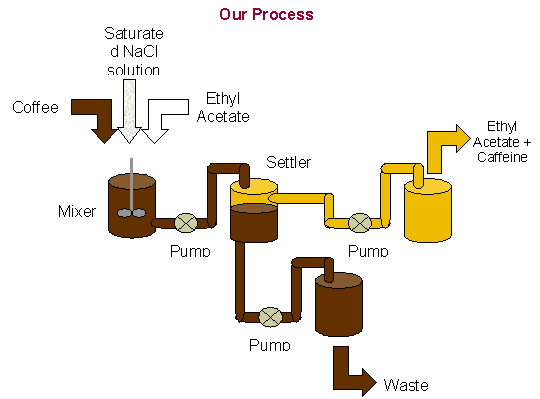
The unit operations we choose to run in the laboratory included mixing,
settling and pumping a variety of materials different places.
A large vessel was required for the mixing of the coffee with the
saturated NaCl solution and the ethyl acetate.
It was placed on a stir plate with a stir bar for 10 minutes.
Once the solution was completely mixed, it was pumped into a smaller
settling vessel. Here, two distinct
layers formed due to the differences in densities. The oily organic layer, which contained the caffeine, was
found on top. This layer was pumped
into another vessel. The remaining
layer was the decaffeinated coffee. This
coffee, in industry, would be purified to remove any remaining decaffeinating
agent. The oily organic layer was
then boiled to remove the decaffeinating agent, ethyl acetate.
Pure caffeine was not obtained at this time due to the fact that the
caffeine was still suspended in the oil. The
oil would need to be evaporated, and then the caffeine would need to be
re-crystallized in order to obtain pure caffeine.
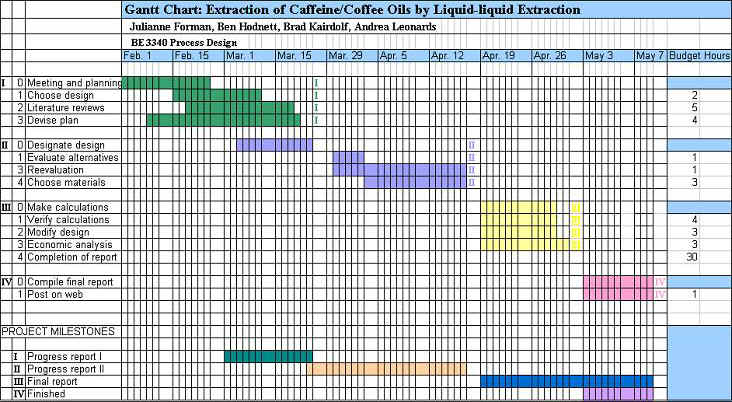
Updated
Gantt Chart
– See Appendix B
Results
and Discussion:
Our experiment demonstrated a successful set-up and was triumphant in
removing caffeine from the coffee, however we did not obtain pure caffeine.
The caffeine was trapped inside the oil instead of binding only with the
ethyl acetate. Further unit
operations would have been required to achieve pure white powder caffeine.
This process proved to be an effective way to remove caffeine from
coffee. On the other hand, a more
effective means may eliminate the oily layer and have the caffeine only in
solution with the ethyl acetate. The oils extracted by the ethyl acetate were an unexpected
problem, but it did not affect the decaffeinating process greatly.
Economic
Analysis:
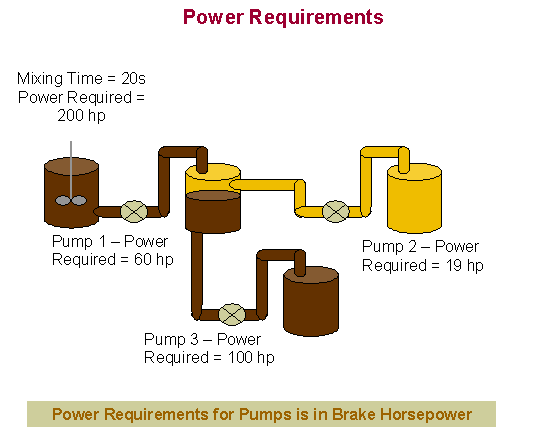
The
equipment used for our unit operations included a mixer and three pumps.
All materials were scaled up using the appropriate formulas and a cost
analysis was performed. The basic
formulas and results are found in the chart below while all the details of the
calculations can be found in Appendix C.
|
|
Scale Up Factors
|
Cost Analysis
|
|
Agitator
|
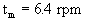
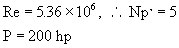
|
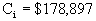
|
|
Pump 1
|
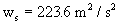
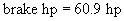
|
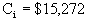
|
|
Pump 2
|
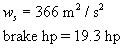
|
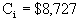
|
|
Pump 3
|
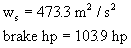
|
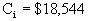
|
|
Total Cost
|
|
$221,440
|
Appendix
Appendix A:
Appendix B
Appendix C
Scale-up
of Agitator
Density of ethyl acetate = 894.5
kg/m³
Dynamic Viscosity of ethyl acetate
= 0.426 x 10-3 Pa·s.
Density of water = 1000 kg/m³
Dynamic Viscosity of water = 0.86 x
10-3 Pa·s
Density of saturated salt solution
(NaCl) = 1100 kg/m³
Dynamic Viscosity of saturated salt
solution = 0.89 x 10-3 Pa·s.
Density of mixture (water/saturated
salt solution) = 1050 kg/m³
Dynamic Viscosity of mixture
(water/saturated salt solution) = 0.9 x 10-3 Pa·s.
Volume of mixture (water/saturated
salt solution) in lab scale = 350 mL.
Volume of ethyl acetate in lab
scale = 100 mL.
Total volume in mixing chamber in
lab scale = 450 mL.
Approximate density of fluid in
mixing chamber = (1050 kg/m³) · (0.777) + (894.5 kg/m³) · (0.222) = 1015
kg/m³.
Approximate dynamic viscosity of
fluid in mixing chamber = (0.9 x 10-3 Pa·s) · (0.777) + (0.426 x 10-3
Pa·s) · (0.222) = 0.795 x 10-3 Pa·s.
Volume of lab scale reactor = 500
mL.
Diameter of reactor = 8 cm = 0.08
m.
Diameter of impeller = 2 in =
0.0508 m.
Speed of lab mixer = 30 rpm = 3.14 rad/s.
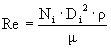 è
Re = 20701 = 2.1 x 104 è
turbulent è
assume Np’ = 5.
è
Re = 20701 = 2.1 x 104 è
turbulent è
assume Np’ = 5.
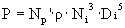 è
P = 0.05 W = scale power.
è
P = 0.05 W = scale power.
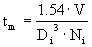 è
tm = 1.9 s.
è
tm = 1.9 s.
Since keeping the time on scale-up is not feasible, we
scaled up our mixing time to 20 s. Using
a new mixing time, we had to calculate a new impeller speed.
Using a scaled-up vessel (3-m radius by 5-m height), a
scaled-up impeller diameter of 2.5 m, and the scaled-up time of 20 s, we were
able to calculate our new Ni.
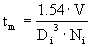 è
Ni = 0.67 rad/s = 6.4 rpm.
è
Ni = 0.67 rad/s = 6.4 rpm.
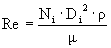 è
Re = 5.36 x 106. Use Np’
= 5.
è
Re = 5.36 x 106. Use Np’
= 5.
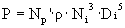 è
P = 149200 W = 200 hp.
è
P = 149200 W = 200 hp.
Cost –
Analysis of Agitator
Our scaled-up agitator will be a single blade Rushton
turbine.
Base cost of 200 hp agitator (dual turbine read from chart)
è
$90,000.
Fmod = 2.0
Fm for single blade turbine = 0.82
CIpresent = 392.7, CIbase = 324
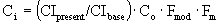 è
$178,897.
è
$178,897.
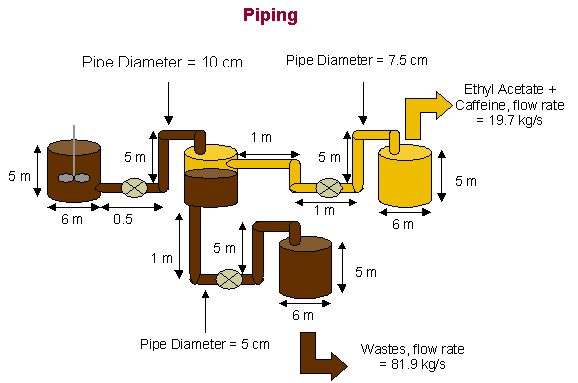
Scale-up of
Pump 1 (from mixer to settler)
The volumetric flow from mixer to settler was selected as
0.1 m³/s. The pipe diameter was
selected as 10 cm = 0.1 m.
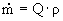 = (0.1 m³/s) · (1015 kg/m³) è
101.5 kg/s
= (0.1 m³/s) · (1015 kg/m³) è
101.5 kg/s
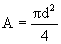 è
0.0078 m³.
è
0.0078 m³.
Q = v · A è
v = 12.73 m/s
Leq = 3 · 35 · 0.1 + 0.5 + 5 = 16.0 m
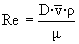 è
Re = 1.6 x 106 è
turbulent
è
Re = 1.6 x 106 è
turbulent
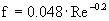 è
f = 0.0275
è
f = 0.0275
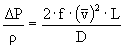 è
142.6 m²/s²
è
142.6 m²/s²
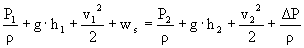 è
ws = 223.6 m²/s²
è
ws = 223.6 m²/s²
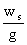 = 22.79 m
= 22.79 m
For the following brake horsepower calculation, we chose
our pump efficiency to be 0.5. This
was done to give an overestimate of cost, rather than an underestimate.
Brake hp =
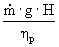 è
45384.7 W è
60.9 hp
è
45384.7 W è
60.9 hp
Cost – Analysis of Pump 1
Our scaled-up pump will be a turbine with a brake
horsepower of 60 hp.
Base cost of turbine = $7000.
Fmod = 1.80
Fm = none
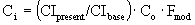 è
$15,272
è
$15,272
Scale-up of
Pump 2 (from settler (ethyl acetate))
The volumetric from mixer to settler was calculated to be
22% of the original flow or 0.022 m³/s. The
pipe diameter was selected as 5 cm = 0.05 m.
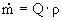 = (0.022 m³/s) · (894.5 kg/m³) è
19.7 kg/s
= (0.022 m³/s) · (894.5 kg/m³) è
19.7 kg/s
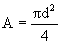 è
0.002 m³.
è
0.002 m³.
Q = v · A è
v = 11.2 m/s
Leq = 5 · 35 · 0.05 + 1 + 4.75 + 1 +5 = 20.5 m
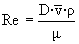 è
Re = 1.2 x 106 è
turbulent
è
Re = 1.2 x 106 è
turbulent
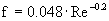 è
f = 0.003
è
f = 0.003
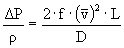 è
301 m²/s²
è
301 m²/s²
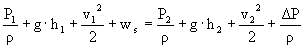 è
ws = 366 m²/s²
è
ws = 366 m²/s²
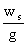 = 37.31 m
= 37.31 m
For the following brake horsepower calculation, we chose
our pump efficiency to be 0.5. This
was done to give an overestimate of cost, rather than an underestimate.
Brake hp =
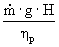 è
14420.8 W è
19.3 hp
è
14420.8 W è
19.3 hp
Cost –
Analysis of Pump 2
Our scaled-up pump will be a turbine with a brake
horsepower of 19 hp.
Base cost of turbine = $4000.
Fmod = 1.80
Fm = none
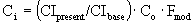 è
$8727
è
$8727
Scale-up of
Pump 3 (from settler (wastage))
The volumetric from mixer to settler was calculated to be
78% of the original flow or 0.078 m³/s. The
pipe diameter was selected as 7.5 cm = 0.075 m.
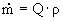 = (0.078 m³/s) · (1050 kg/m³) è
81.9 kg/s
= (0.078 m³/s) · (1050 kg/m³) è
81.9 kg/s
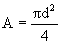 è
0.0044 m³.
è
0.0044 m³.
Q = v · A è
v = 17.6 m/s
Leq = 3 · 35 · 0.075 + 1 + 5 = 13.875 m
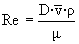 è
Re = 1.54 x 106 è
turbulent
è
Re = 1.54 x 106 è
turbulent
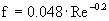 è
f = 0.0278
è
f = 0.0278
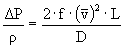 è
318 m²/s²
è
318 m²/s²
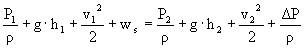 è
ws = 473.3 m²/s²
è
ws = 473.3 m²/s²
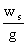 = 48.25 m
= 48.25 m
For the following brake horsepower calculation, we chose
our pump efficiency to be 0.5. This
was done to give an overestimate of cost, rather than an underestimate.
Brake hp =
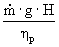 è
77531.9 W è
103.9 hp
è
77531.9 W è
103.9 hp
Cost – Analysis of Pump 3
Our scaled-up pump will be a turbine with a brake
horsepower of 100 hp.
Base cost of turbine = $8500.
Fmod = 1.80
Fm = none
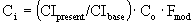 è
$18,544
è
$18,544
Total
Cost = $221,440
Our Settling Tank

Our Mixing Tank

Chemicals
of Interest
References
Doran, Pauline M. Bioprocess
Engineering Principles. Academic Press, San Diego: 1995.
http://www.sciam.com/0697issue/0697working.html
http://antoine.frostburg.edu/chem/senese/101/matter/faq/decaffeinating-coffee.shtml
http://www.cariboucoffee.com/aco.cfm?ct=a
Walker, Terry. BE 3340: Process Design in Biological Engineering.
2002.
Garrigues, Jose M., Zouhair
Bouhsain, Salvador Garrigues and Miguel de la Guardia. “Fourier transform
infrared determination of caffeine in roasted coffee samples”, Fresenius
Journal of Analytical Chemistry.
Mikalsen, Anita, Bard Bertelsen
and Magna Arve Flaten. “Effects of caffeine, caffeine-associated stimuli, and
caffeine-related information on physiological and psychological arousal”, Psychopharmacology.
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()









